Ex Vivo Model
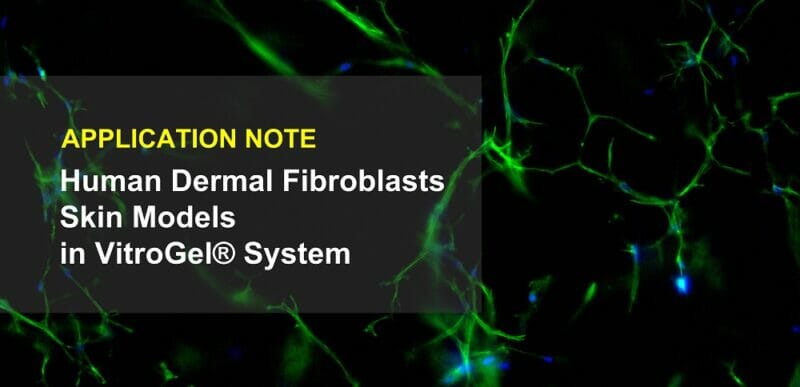
Lymphomoids: A Tissue-based Ex Vivo Culture System for Lymphoma Therapy Screening
VitroGel® RGD High Concentration
RGD modified – tunable, xeno-free hydrogel – high concentration (3 mL kit)
VitroGel RGD High Concentration
VitroGel® RGD High Concentration is a tunable, xeno-free (animal origin-free) hydrogel system modified with cell adhesive peptide RGD to promote cell attachment and cell-matrix interactions during the 3D cell culture. VitroGel RGD High Concentration comes with VitroGel Dilution Solution to adjust the final hydrogel strength from 10 to 4000 Pa.
VitroGel High Concentration hydrogels are our xeno-free, tunable hydrogels for researchers wanting full control to manipulate the biophysical and biological properties of the cell culture environment. The tunability of the hydrogel gives the ability to create an optimized environment for cell growth. The hydrogel system has a neutral pH, transparent, permeable and compatible with different imaging systems. The solution transforms into a hydrogel matrix by simply mixing with the cell culture medium. No cross-linking agent is required. Cells cultured in this system can be easily harvested with our VitroGel® Cell Recovery Solution. The hydrogel can also be tuned to be injectable for in vivo studies.
From 3D cell culture, 2D cell coating to animal injection, VitroGel makes it possible to bridge the in vitro and in vivo studies with the same platform system.
Mix & Match – 3D Cell Culture Your WAY!
![]() Unique to VitroGel High Concentration hydrogels is the ability to tailor create a multi-functional hydrogel by blending different types of VitroGel. VitroGel® RGD High Concentration can be “mix & matched” with other VitroGel High Concentration hydrogels such as VitroGel® IKVAV, VitroGel® YIGSR, VitroGel® MMP, and VitroGel® COL to create a customized multi-functional hydrogel. Using this flexible and powerful hydrogel system, scientists customize their 3D culture micro-environment for different applications.
Unique to VitroGel High Concentration hydrogels is the ability to tailor create a multi-functional hydrogel by blending different types of VitroGel. VitroGel® RGD High Concentration can be “mix & matched” with other VitroGel High Concentration hydrogels such as VitroGel® IKVAV, VitroGel® YIGSR, VitroGel® MMP, and VitroGel® COL to create a customized multi-functional hydrogel. Using this flexible and powerful hydrogel system, scientists customize their 3D culture micro-environment for different applications.
Specifications
| Contents | VitroGel® RGD High Concentration, 3 mL VitroGel® Dilution Solution, 50 mL |
| Hydrogel Formulation | Xeno-free tunable hydrogel modified with RGD peptide. |
| Use | Good for adhesion cells or cells requiring stronger cell-matrix interactions. |
| Mix & Match | Can be blended with other versions of VitroGel concentrated hydrogels to create a custom multi-functional matrix. |
| Operation | Room temperature |
| Hydrogel Strength | 10 to 4,000 Pa of G’ depending on dilution ratio. Dilute with VitroGel Dilution Solution (TYPE 1 or TYPE 2) for different concentrations. |
| pH | Neutral |
| Color | Transparent |
| Cell Harvesting | VitroGel Organoid Recovery Solution 5-15 min cell recovery |
| Injectable | Injectable hydrogel |
| Storage | Store at 2-8°C. Ships at ambient temperature |
| Number of Uses | Dilution ratio: 1:2 = 225 uses at 50 µL per well 1:3 = 300 uses at 50 µL per well 1:5 = 450 uses at 50 µL per well |
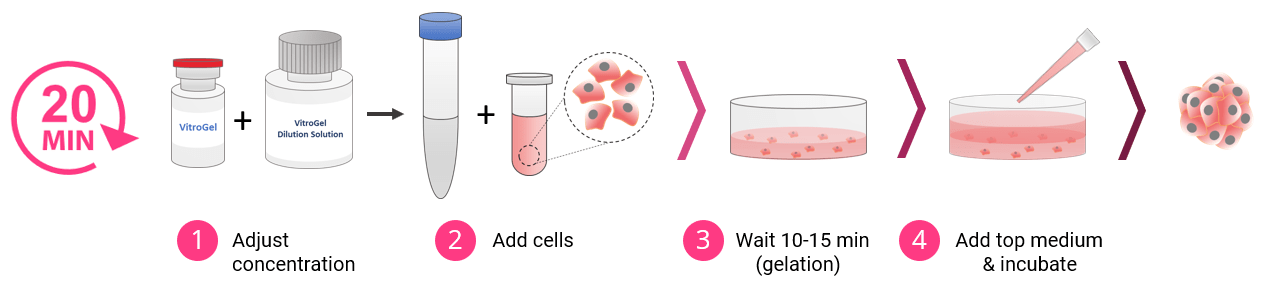
3D Cell Culture Process in 20 Minutes
VitroGel High Concentration hydrogels are easy to use. There is no cross-linking agent required. Work confidently at room temperature.
Tunable Hydrogel Strength
Simply diluting the hydrogel controls the gel strength.
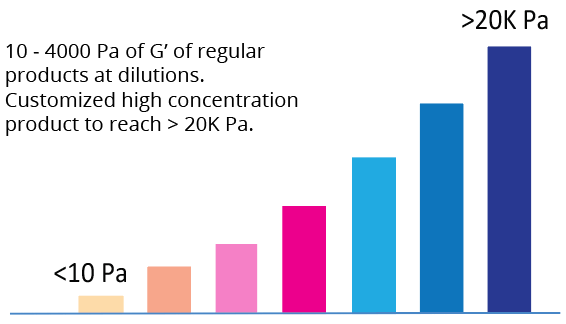
Protocols / Handbooks / Resources
Video Protocols & Demonstrations
Webinars
Research Highlights
Application Notes
Data and References
Cell Type Behavior Reference Table – VitroGel RGD
Multiple studies have made use of RGD hydrogel in different tissue and cell types. RGD is commonly used as an immobilized, adhesive ligand in 3D hydrogels that allows researcher to study many different cellular processes and behaviors in normal physiological and pathological contexts.
| Cell Type | Behavior |
|---|---|
| Goat bone marrow stromal cells | Promoted osteogenic differentiation |
| Rat bone marrow stromal cells | Promoted osteogenic differentiation |
| Rat osteoblasts | Increased cell attachment and spreading |
| Cell Type | Behavior |
|---|---|
| Biphasic synovial sarcoma SYO-1 | Cell proliferation and cell matrix interactions |
| Bone OSA 1777 | Cell proliferation and cell matrix interactions |
| Breast 4T1 | Cell proliferation, division, migration, and invasion |
| Breast AU-565 | Cell proliferation and cell matrix interactions |
| Breast Cancer MCF-7 | Cell proliferation, intercellular connections |
| Breast E0771 | Cell proliferation and cell matrix interactions |
| Breast MDA-MB-231 | Cell proliferation, division, migration, and invasion |
| Breast T47D | Cell proliferation, division, migration, and invasion |
| Colorectal adenocarcinoma DLD-1 cells | Cell proliferation and cell matrix interactions |
| Epithelial ovarian OV-MZ-6 | Promoted spheroid formation and proliferation |
| Epithelial ovarian SKOV-3 | Promoted spheroid formation and proliferation |
| Fuji Cells | Cell proliferation and cell matrix interactions |
| Glioblastoma SF 268 | Cell proliferation and cell matirx interaction |
| Glioblastoma SF 295 | Cell proliferation and cell matirx interaction |
| Glioblastoma SNB75 | Cell proliferation and cell matirx interaction |
| Glioblastoma U-251 MG | Cell proliferation and cell matirx interaction |
| Glioma U87-MG | Increased cell spreading and actin stress fiber assembly |
| Glioma U87-MG | Cell proliferation and cell matirx interaction |
| Glioma U373-MG | Increased cell adhesion duration and migration (on higher stiffness) |
| HEK 293 | Cell proliferation and cell matrix interactions |
| Huaman colon carcinoma HCT-8 | Cell proliferation and cell matirx interaction |
| Human colorectal carcinoma HCT 116 | cell proliferation, cell survival, and intercelluar networking |
| Human pancreatic cancer PANC-1 | cell proliferation and cellular interactions |
| Insulinoma ins-1 (Rat) | Cell proliferation and cell matrix interactions |
| Liver carcinoma HepG2 | Cell proliferation and cell matirx interaction |
| Melanoma Cells | Cell proliferation and cell matrix interactions |
| Ovarian carcinoma OVCAR-3 | Cell proliferation and invasion |
| Primary glioblastom U87 | cell proliferation and cellular interactions |
| Prostate adenocarcinoma LNCaP | Increased cell attachment |
| Prostate CRPC | Cell proliferatin and invasion |
| Prostate DU145 | Cell proliferation and invasion |
| Prostate PC3 | Cell proliferation and invasion |
| Cell Type | Behavior |
|---|---|
| Bovine chondrocytes | Increased cell viability and proliferation |
| Bovine chondrocytes | Promoted cell attachment, viability, and stress fiber formation |
| Human chondrocytes | Promoted cell viability and proliferation |
| Cell Type | Behavior |
|---|---|
| Fibroblast NIH3T3 | Promoted cell spreading |
| Fibroblasts NIH3T3 | Increased directional cell migration toward gradient |
| Human dermal fibroblasts | Promoted cell survival and spreading |
| Human dermal fibroblasts | Increased cell adhesion and proliferation |
| Human foreskin fibroblasts | Promoted cell spreading |
| Cell Type | Behavior |
|---|---|
| A549 cells | Cell proliferation and invasion |
| MCF-12A | Cell proliferation and invasion |
| Mouse ovarian follicle cells | Cell proliferation and invasion |
| Cell Type | Behavior |
|---|---|
| Beta TC3 Cells | Cell proliferation and cellular interactions |
| Cell Type | Behavior |
|---|---|
| Human embryonic kidney HEK293 | Promoted spheroid formation |
| Madin-Darby Canine Kidney | Promoted formation of structured epithelial cysts |
| Cell Type | Behavior |
|---|---|
| Human hepatocytes | Increased number of filopodia and synthesis of albumin |
| Mouse hepatocytes | Promoted cell viability |
| Cell Type | Behavior |
|---|---|
| Alveolar epithelial A549 | Inhibited cell detachment |
| Alveolar epithelial RLE-6TN | Increased cell attachment and mesenchymal differentiation |
| Cell Type | Behavior |
|---|---|
| Mouse skeletal myoblasts | Promoted cell attachment, proliferation, and myofibril formation |
| Myoblasts C2C12 | Promoted cell proliferation and differentiation |
| Cell Type | Behavior |
|---|---|
| Chick dorsal root ganglion cells | Increased neurite length, neurite outgrowth, and neurite number |
| In vivo lesioned rat cortex | Supported angiogenesis and inhibited glial scars |
| In vivo lesioned rat spinal cord | Supported angiogenesis and axon regeneration |
| Cell Type | Behavior |
|---|---|
| Human embryonic stem cells | Increased retinal pigmented epithelium and optic vesicle development |
| Human iPSC | Cell proliferation, and cell matrix interactions |
| Human mesenchymal stem cells | Increased cell viability |
| Mouse embryonic stem cells | Promoted endothelial cell differentiation |
| Mouse mesenchymal stem cells | Promoted cell spreading and migration |
| Rat mesenchymal stem cells | Increased cell adhesion and spreading |
| Rat mesenchymal stem cells | Promoted cell attachment and differentiation |
| Cell Type | Behavior |
|---|---|
| Human aortic smooth muscle cells | Promoted cell attachment |
| Human umbilical vein endothelial cells | Increased cell adhesion, proliferation, migration, and angiogenesis |
| Human umbilical vein endothelial cells | Increased cell adhesion and proliferation |
| Rat neonatal cardiac | Promoted cell attachment and tissue regeneration and prevented apoptosis |
Data
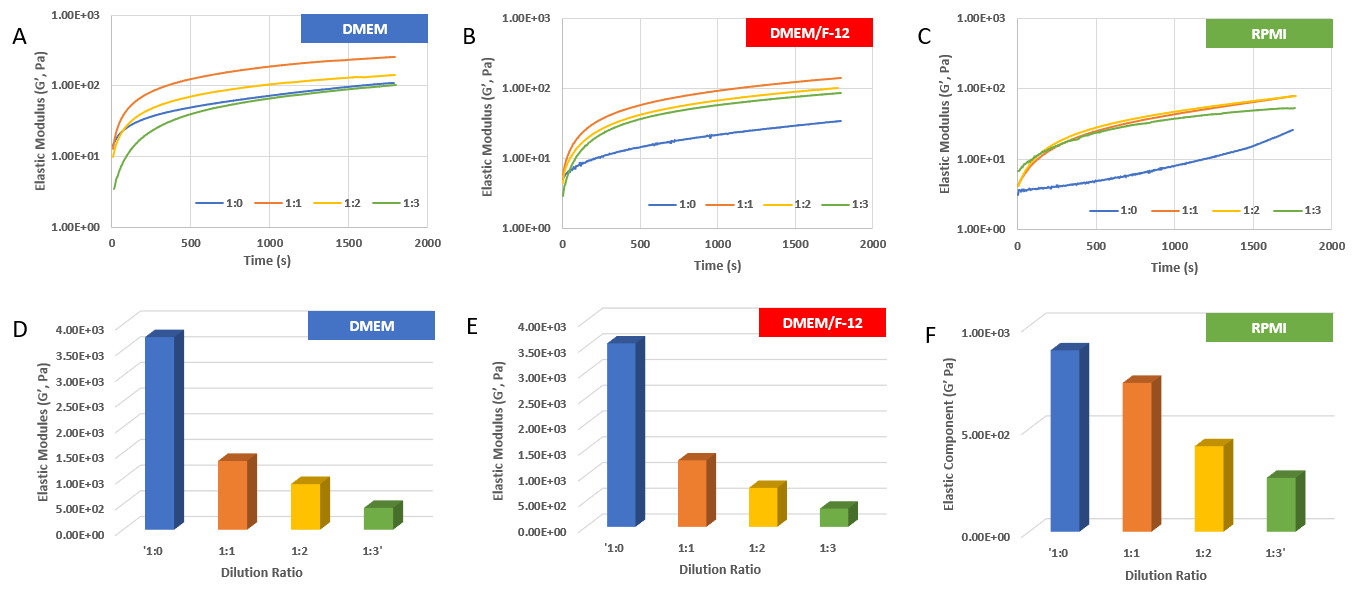
Figure 1. Rheological properties of VitroGel RGD with DMEM medium.
A) – C) The gel formation curve after mixing with DMEM (A), DMEM/F-12 (B), and RPMI (C) media. VitroGel RGD was diluted at 1:0,1:1, 1:2 and 1:3 (v/v) with VitroGel Dilution Solution (Type 1) and then mix with media at 4:1 (v/v) ratio; D) – F) The gel strength after 24 hrs incubation in DMEM (D), DMEM/F-12 (E), and RPMI (F) media. The hydrogel was prepared as method A and incubated at 37°C CO2 incubator for 24 hrs before the rheological test. (10 ~ 4000 Pa of G’ of regular products at dilutions. Customized high concentration product to reach over 20K Pa)
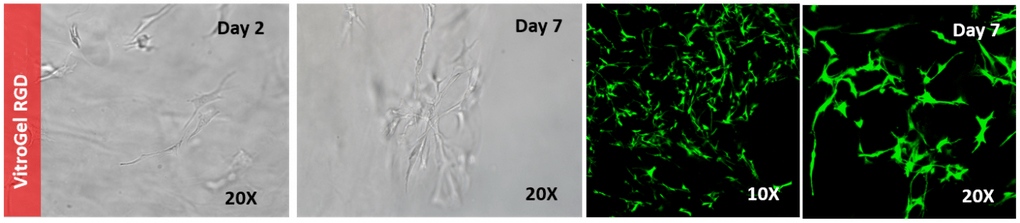
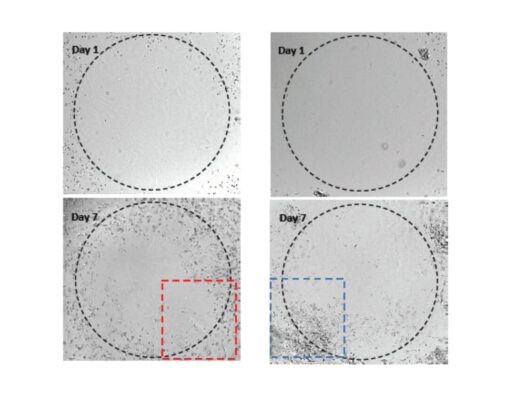
Figure 2. 3D culture of OP9 cells in VitroGel RGD.
Hydrogel was prepared at 1:3 dilution with VitroGel Dilution Solution (Type 1). The images were taken on days 2 and 7. VitroGel RGD shows support for OP9 cell proliferation and cell-cell communication. The stronger cell-matrix interactions help the cells to form the cell-networking structure.
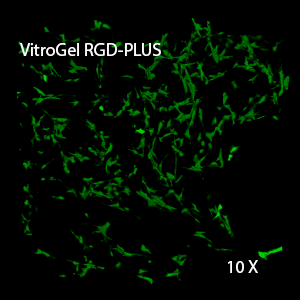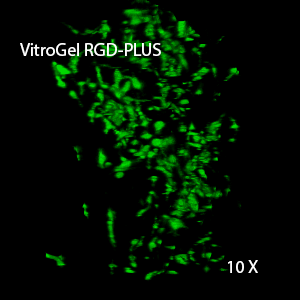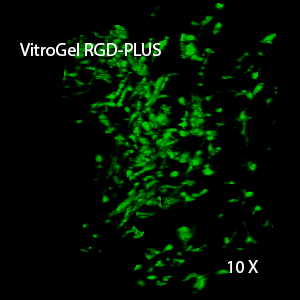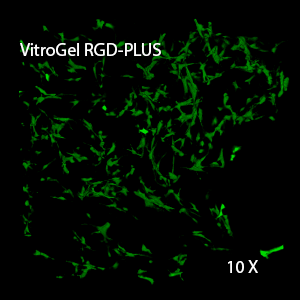
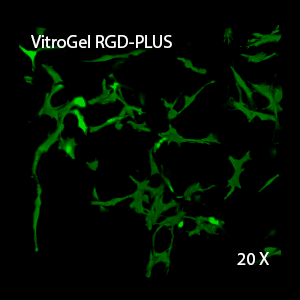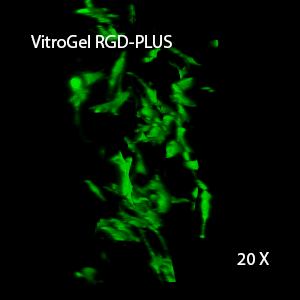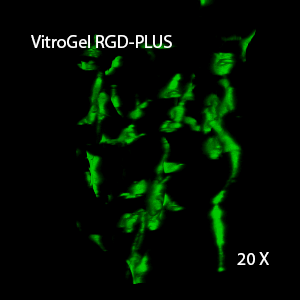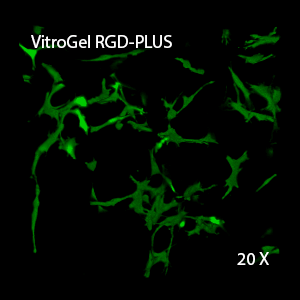
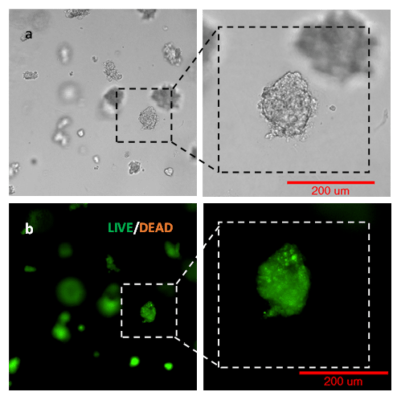
Figure 3. 3D view of OP9 cells growth in VitroGel RGD.
Cell networking structure formed in VitroGel RGD.
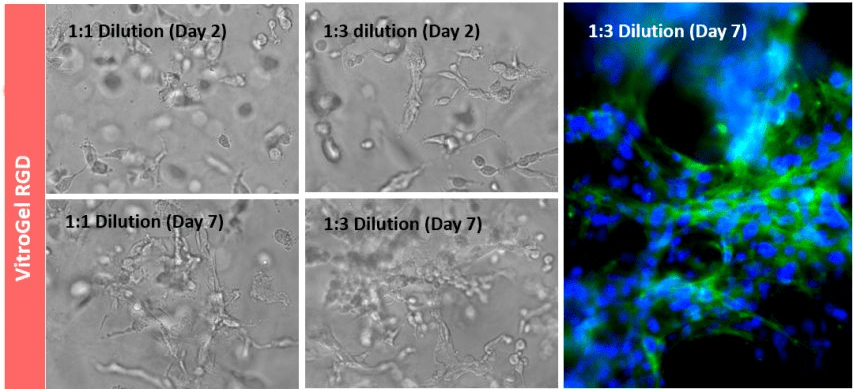
Figure 4. 3D culture of U87-MG cells in VitroGel RGD.
Cells can grow in 3D hydrogel at 1:1 and 1:3 dilution of VitroGel RGD. U87-MG cells shows cell networking structure and the cell morphology in VitroGel RGD indicating a cell-cell and cell-matrix interaction.
References/Publications
- Mokhtari, R. B., Sampath, D., Eversole, P., Ong, M., Bosykh, D. A., Gandhi T.K. Boopathy, Sivakumar, A., Wang, C., Kumar, R., Yeong, J., Karasik, E., Foster, B. A., Yu, H., Ling, X., Wu, W., Li, F., Ohler, Z. W., Brainson, C. F., Goodrich, D. W., & Hong, W. (2025). An Agrin–YAP/TAZ Rigidity Sensing Module Drives EGFR‐Addicted Lung Tumorigenesis. Advanced Science. https://doi.org/10.1002/advs.202413443
- Sun, P., Xu, H., Guo, C., Yang, L., Zhang, X., Lu, B., Chen, L., & Huang, J. (2025). TMEM115 as an Oncogenic and Immunological Biomarker in Hepatocellular Carcinoma. Liver International : Official Journal of the International Association for the Study of the Liver, 45(4), e70048. https://doi.org/10.1111/liv.70048
- Trucco, D., Gibney, R., Vannozzi, L., Lisignoli, G., Kelly, D. J., & Ricotti, L. (2025). Reinforcement of injectable hydrogels through melt electro-written structures: Influence of shape and pore size on the injection force. Journal of Materials Research and Technology, 36, 358–368. https://doi.org/10.1016/j.jmrt.2025.03.133
- Gabusi, E., Lenzi, E., Manferdini, C., Dolzani, P., Columbaro, M., Saleh, Y., & Lisignoli, G. (2022). Autophagy Is a Crucial Path in Chondrogenesis of Adipose-Derived Mesenchymal Stromal Cells Laden in Hydrogel. Gels, 8(12), 766. https://doi.org/10.3390/gels8120766
- Hao, X., Zhang, S., Li, P., Huang, J., Yuan, Z., & Tan, J. (2022). Amniotic membrane extract-enriched hydrogel augments the therapeutic effect of menstrual blood-derived stromal cells in a rat model of intrauterine adhesion. Biomaterials Advances,142, 213165. https://doi.org/10.1016/j.bioadv.2022.213165
- Ding, J., et al. (2022). RGD-Hydrogel Improves the Therapeutic Effect of Bone Marrow-Derived Mesenchymal Stem Cells on Phosgene-Induced Acute Lung Injury in Rats Computational Intelligence and Neuroscience. https://www.hindawi.com/journals/cin/2022/2743878/
- Manferdini, C., et al. (2022). RGD-Functionalized Hydrogel Supports the Chondrogenic Commitment of Adipose Mesenchymal Stromal Cells Gels. https://www.mdpi.com/2310-2861/8/6/382
- Fen, et al.(2022) Optimization of Three-Dimensional Culture Conditions of HepG2 Cells with Response Surface Methodology Based on the VitroGel System. Biomedical and Environmental Sciences,(35,8), 688-698. https://www.frontiersin.org/articles/10.3389/fimmu.2022.914381/full
- Powell K. Adding depth to cell culture. Science, 356(6333), 96–98. https://doi.org/10.1126/science.356.6333.96